PLANO, Texas, April 25, 2022 /PRNewswire/ — Bimini Health Tech (“Bimini” or the “Company”), a diversified global medical aesthetics and regenerative therapy company, announced today that it has received approval from Canada’s Medical Devices Directorate to market the Company’s Dermapose family of microsizing syringe products, including the Dermapose Refresh Microsizing Syringe and the Autopose Restore Microsizing Syringe in Canada.
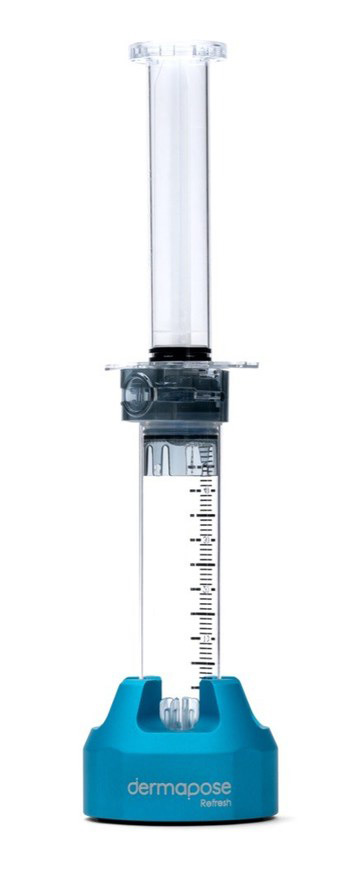
Bimini will market the complete portfolio of Dermapose® and AutoPose™ Microsizing Syringe products in Canada through its local partners Suneva Medical, a leading provider of Aesthetic and Regenerative solutions, and Arthrex®, a global leader in minimally invasive orthopedic technology.
“This is a great achievement for Bimini Health Tech team as we drive the global adoption of the line of Dermapose products. The Canadian Medical Devices Directorate’s approval allows us to launch the Dermapose family of products into the Canadian market. With the inclusion of Dermapose, we continue the expansion of our portfolio of autologous products available in Canada, including the Puregraft® Fat Grafting System, recognized as the gold-standard in fat transfer, and Healeon HD PRP®, marketed under two private labels,” said Brad Conlan, CEO of Bimini Health Tech. “Plastic surgery and non-aesthetic procedures are shifting towards more natural approaches like autologous fat transfer procedures and the demand for predictable, outcome-based technology has increased.” Brad continued, “Dermapose allows the physician to harvest and process the patient’s adipose tissue in a safe, consistent method; and assures the patient that the graft they are receiving is natural with a high retention rate post-transplant. Our commitment is to the patient, the physician, and their trust in Bimini Health Tech’s products.”
Dermapose is the newest cutting-edge fat transfer technology from the experts of Puregraft, dedicated to volumization and rejuvenation. It is a unique all-in-one device designed to purify, microsize and reinject a patient’s fat simply and elegantly.
Learn more about Dermapose at www.dermapose.com and Bimini Health Tech at www.biminihealthtech.com.
About Bimini Health Tech
Bimini Health Tech is a global leader in the medical aesthetics and regenerative market. The Bimini Health Tech portfolio includes Puregraft, Healeon, Dermapose and Kerastem. The company develops and commercializes innovative products that are elegant in their simplicity, yet impactful and proven in their aesthetic, reconstructive and therapeutic benefit. Since 2013, they have been developing innovative products to provide premium aesthetic and regenerative care options to consumers and physicians alike.
About Dermapose Refresh
Dermapose Refresh is a Suction Lipoplasty system. The Dermapose Refresh is a sterile medical device intended for the closed-loop processing of lipoaspirate tissue in medical procedures involving the harvesting, concentrating and transferring of autologous adipose tissue harvested with a legally marketed lipoplasty system. The device is intended for use in the following surgical specialties when the transfer of harvested adipose tissue is desired: orthopedic surgery, arthroscopic surgery, neurosurgery, gastrointestinal and affiliated organ surgery, urological surgery, general surgery, gynecological surgery, thoracic surgery, laparoscopic surgery, and plastic and reconstructive surgery when aesthetic body contouring is desired. Only legally marketed accessory items, such as syringes, should be used with the system. If harvested fat is to be transferred, the harvested fat is only to be used without any additional manipulation. See the Instructions for Use that accompanies the product for important Warnings, Precautions, and Directions.